Polyfuranoates
Bio-based Poly(alkylene-2,5-furan dicarboxylate) Esters
Properties
Polyfuranoates are a relative new class of semi-amorphous polymers which can be produced by polycondensation. They are semi-aromatic, fully transparent thermoplastics that can be easily molded and thermoformed. A polyfuranoate that has gained a lot of attention and that is currently investigated for commercial use is poly(ethylene-2,5-furanoate) (PEF). It is one of the most extensively studied biobased thermoplastic polyesters that is truly biodegradable and biocompatible. It can be considered the furan-based analogue to poly(ethylene terephthalate) (PET) and could replace conventional polyesters in many applications. In fact, it is often considered the next-generation PET. Compared to PET, PEF has greatly improved barrier properties.1 For example, it has six to10 times lower O2, two to four times lower moisture and four to six times lower CO2 permeability than comparable PET film. Thus, excellent barrier properties can be achieved without the need of extra barrier layers such as PVDC and EVOH.
PEF has many other attractive thermal and mechanical properties including high strength and high puncture toughness, good heat resistance, as well as lower melting point (211°C vs. 247°C) and higher glass transition temperature (85°C vs. 76°C) than comparable PET, and thus has more attractive thermal properties.3
PEF can be processed with conventional polymer extrusion and injection molding equipment at temperatures from 240 °C up to over 300°C. Its melt viscosity at around 270°C is similar to that of comparable PET resin, while molten PEF is less viscous at higher temperatures and more viscous at lower temperatures.
Other biodegradable polyfuranoates based on FDCA that are currently investigated include poly(2-methyl-1,3-propylene-2,5-furanoate) (PMePF), poly(1,4-cyclohexanedimethylene-2,5-furanoate) (PCHDMF), and poly(isosorbide-2,5-furanoate) (PIsF) among several others. These polyesters could replace conventional polyesters such as PET, poly(trimethylene terephthalate) (PTT) and poly(butylene terephthalate) (PBT) in many applications.
Synthesis
PEF can be produced by polycondensation of ethylene glycol and 2,5-furandicarboxylic acid (FDCA) or its methyl ester 2,5-dimethylfuran-dicarboxylate
(DMFD). Both building blocks can be derived from sugar via a
chemical catalytic dehydration of sugar to 5-hydroxymethylfurfural (5-HMF) followed by oxidation (FDCA) and esterification with methanol
anhydride (DMFD).
PEF is typically prepared through a two-stage melt process (esterification and polycondensation). In the first stage,
DMFD and ethylene glycol at a molar ratio of diester/diol = ½ are esterified in the presence of a catalyst. The reaction is carried out at
temperatures between 160°C - 190°C. In a second stage (polycondensation), the temperature is gradually raised to 230°C. After
the polycondensation is completed, PEF is removed from the reactor, milled, and washed with methanol.5
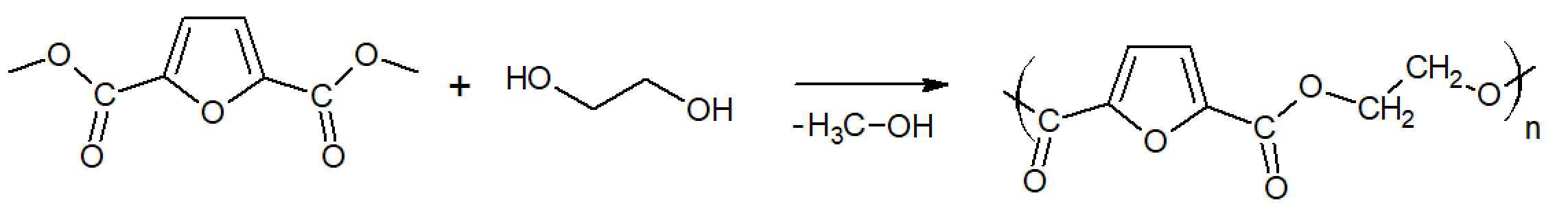
| Polyfuranoates | Structure of Repeat Unit | Properties2-5 |
| Poly(2-methyl-1,3-propylene-2,5-furanoate) (PMePF) |
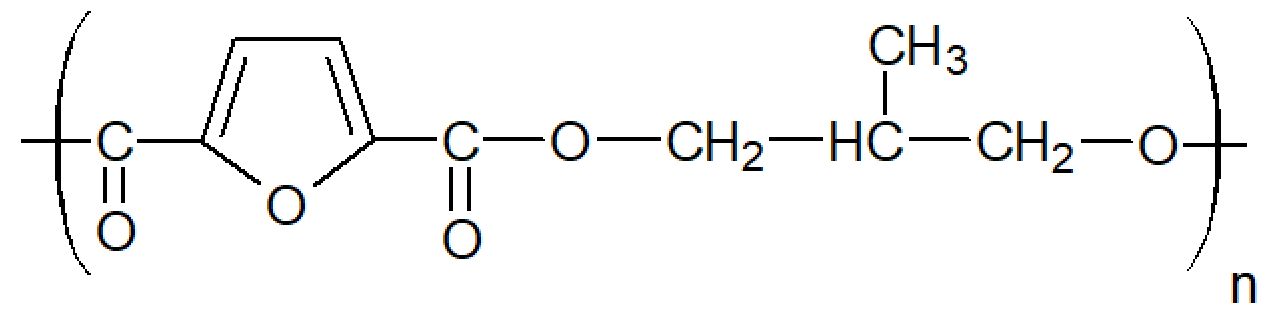
| Tg ≈ 53 °C Tm ≈ N/A |
| Poly(1,4-cyclohexanedimethylene-2,5-furanoate) (PCHDMF) |
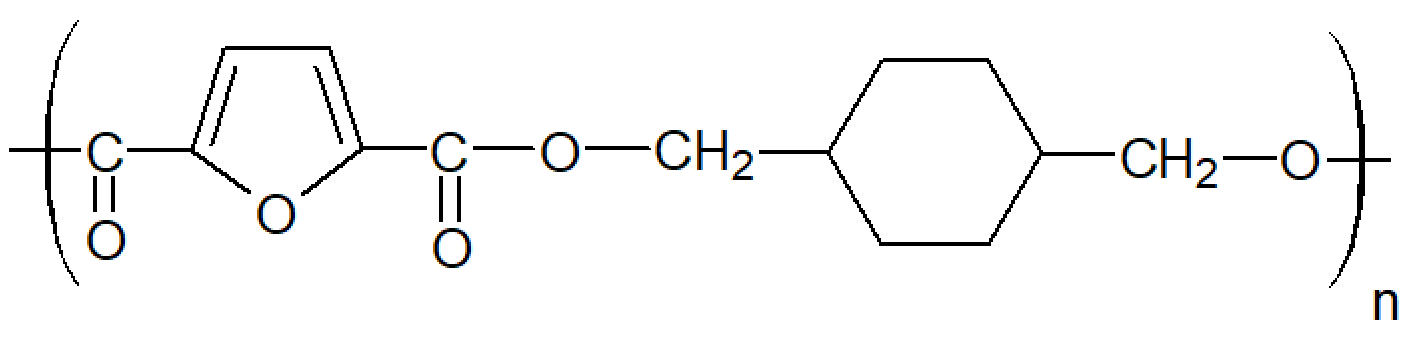
| Tg ≈ 74 °C Tm ≈ 262°C |
| Poly(ethylene-2,5-furanoate) (PEF) |
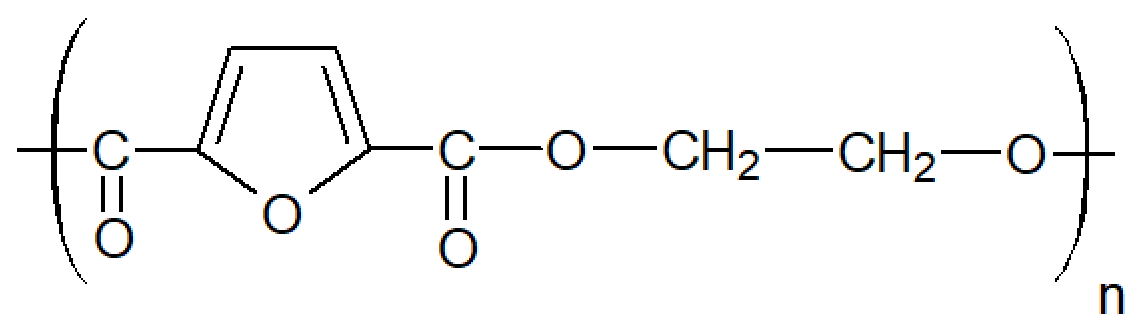
| Tg ≈ 85°C Tm ≈ 210 - 220°C |
COMMERCIAL Biobased PEF
FDCA is currently produced on a pilot scale by Corbion and by a joint venture of Avantium, Synvina, and BASF.
APPLICATIONS
PEF is an interesting alternative to polyethylene terephthalte (PET). Since it is partially or fully produced from renewable feedstocks, it reduces the carbon footprint. Potential applications include fibers, films as well as food and beverage containers, in particular blow-molded bottles for carbonated soft drinks and alcoholic beverages.
The market share of biobased PEF is currently (2018) rather small but is expected to grow significantly.6
References and Notes
- The improved gas barrier properties of PEF compared to PET are unexpected due to PEF’s higher free volume. Burgess et al.2 concluded that the lower permeability is the result of the suppressed furan ring-flipping whereas the active phenyl rings of PET can easily flip, thereby reducing β relaxation motions and diffusion in PEF due to the energy penalty associated with the furan ring rotation and ring polarity.
- S.K. Burgess, J.E. Leisen, B.E. Kraftschik, C.R. Mubarak, R.M. Kriegel, and W.J. Koros, Macromolecules, 47 (4), pp 1383–1391 (2014)
- L. Maini, M. Gigli, M. Gazzano,N. Lotti, D.N. Bikiaris and G.Z. Papageorgiou, Polymers 10, 296 (2018)
- Z. Terzopoulou, N. Kasmi, V. Tsanaktsis, N. Doulakas, D.N. Bikiaris,D.S. Achilias and G.Z. Papageorgiou, Materials, 10, 801 (2017)
- N. Kasmi, M. Majdoub, G.Z. Papageorgiou and D.S. Achilias, Polymers, 9, 607 (2017)
- According to Grand View Research, the global demand for polyethylene furanoate (PEF) is expected to reach almost 17,000 tons by 2022.
- Avantium has a strategic partnerships with Danone, Coca-Cola and ALPLA for developing and commercializing bio-based PEF.
- FFDCA and PEF is currently produced on a pilot scale by a joint venture of Avantium, Synvina, and BASF. To ensure optimal product quality Synvina plans to extend the pilot phase by 24 to 36 months.